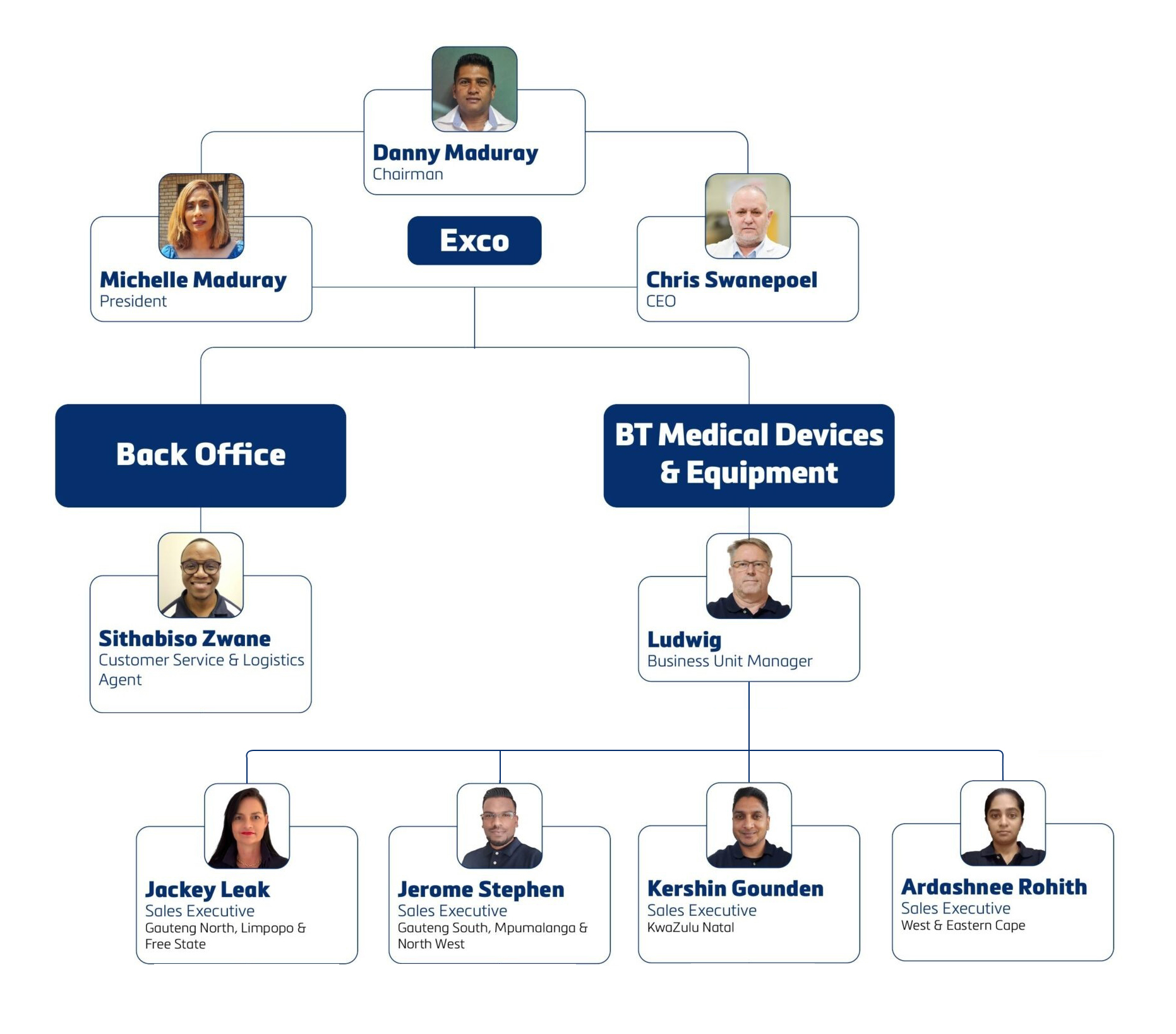
BT Medical Group:
Pioneering Healthcare Innovations
Better Outcomes, Lower Costs
Procurement and supply of innovative medical devices and equipment.
Our drive is to improve clinical outcomes whilst lowering healthcare costs.